Eligibility criteria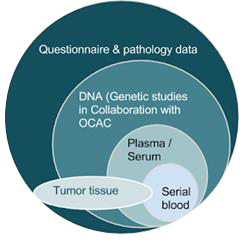
Required
- All cohorts must have prospective ovarian cancer outcomes
- All cohorts must have basic epidemiologic data including oral contraceptive use and parity
Optional
- Constitutional DNA (OCAC)
- Blood
- Serial blood (biomarker)
- Histology data
- Tissue
OC3 Cohorts
Adventist Health Study II (AHS2)
Black Women's Health Study (BWHS)
Breast Cancer Detection Demonstration Project Follow-up Study (BCDDP)
California Teacher’s Study (CTS)
Campaign against Cancer and Heart Disease (CLUE II)
Canadian Study of Diet, Lifestyle, and Health (CSDLH)
Cancer Prevention Study II (CPSII)
European Prospective Investigation into Cancer Project (EPIC)
Finnish Maternity Cohort (FMC)
Generations Study (formerly Breakthrough Generations Study, BGS)
Iowa Women's Health Study (IWHS)
Melbourne Collaborative Cohort Study (MCCS)
Multiethnic Cohort Study (MEC)
Netherlands Cohort Study (NLCS)
NIH-AARP Diet and Health Study (AARP)
Northern Sweden Health and Disease Study (NSHDS)
Nurses’ Health Study II (NHSII)
NYU Women’s Health Study (NYUWHS)
Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO)
Singapore Chinese Health Study (SCHS)
Southern Community Cohort Study (SCCS)
Swedish Mammography Cohort (SMC)
Vitamins and Lifestyle Study (VITAL)
Women's Health Initiative (WHI)
